Welcome to the TPA NETWORK Research Consortium
a Non-Profit, Industry-Wide Healthcare Research Initiative that helps
Providers Evaluate and Assess New Medical Technologies and Innovations,
Plan Participants Obtain Leading-Edge Health Care,
Scientists Advance Medical Research, and
Payers Reduce Risk
Technological innovation is an essential element of modern American medicine however, studies show that new medical technologies account for as much as 30% of the escalation in healthcare costs over time: this makes gaining “control” of the use of technology one of the most effective means by which to contain healthcare costs. Unfortunately, this objective has been fraught with economic, social and bioethical challenges as there are more powerful incentives to develop and diffuse technology than there are ones to manage it. Moreover, even with this knowledge at hand, little research is done that focuses on helping Payers and Plan Sponsors evaluate new healthcare innovations...leaving them to operate in the dark. We want to change this…
The Research Consortium's mission is to vet new medical technologies and innovations to accelerate their adoption to cut the time it takes to get them from the workbench into widespread clinical use by...which now takes 15+ years on average. We do this by conducting research of value to stakeholders that focuses on cost-effectiveness, ROI, value, health outcomes, financial toxicity, patient satisfaction...and other metrics that are not addressed by scientific research that is intended to prove efficacy. This type of translational research is of critical value to Payers as it provides them with evidence-based, real-world support with which to adopt new and emerging innovations that have been vetted and deemed worthy of coverage by their health plans.
As a byproduct of our work, we help Plan Participants access cutting-edge medical treatments and provide Medical Researchers with valuable data and clinical study participants. In the future, funding generated from these activities will provide us with a source of long-term financial sustainability that will enable us to conduct our research at no cost to Payers or Plan Participants.
Lastly, we believe that measuring an organization’s accomplishments is as important than measuring its profits. As such, we are chartered and operated as a non-profit corporation with the intent of one day becoming a Certified B-Corporation that places doing social good ahead of making money. You can learn more about why we feel this way here.



We are an Independent Non-Profit Research Collaborative Focused on Evaluating New Healthcare Technologies and Innovations for Plan Sponsors...
We Enable Plan Participants to Contribute to Medical Research and Benefit from Sharing Their Experiences, Opinions and Health Data, if They Wish...
We Help Patients Access the Latest Medical Technologies, Treatments and Medical Experts with our Clinical-Research-as-a-Care-Option Programs...
To learn more about our solutions for ERISA Plan Sponsors, Stop-Loss Carriers, Plan Participants, etc., click here:
What We Do...
Today’s Healthcare Environment is Characterized by Profound Disruption, Occurring at the Hands of Young, Industry Outsiders
The Price-Impact of Medical Technology and Innovation
New medical technologies and emerging health innovations account for ~ 30% of the unsustainable increase in healthcare costs over time. Many however, are not very effective or are redundant; don't improve quality of care or medical outcomes; or are not a "value" vs. the standard of care.
Rapid Movement to
Value-Based Health Care
The move from the broken fee-for-service model to an unfamiliar/undefined value-based one is occurring at a very rapid pace. Long-established and deep-rooted norms, distinctions and roles among and between TPAs, brokers, providers, etc. are being challenged and causing a lot of agita.
Record-Setting Investment in Disruptive Outsiders
A record-setting level of investment in technologies, solutions, services and business models is occurring...all aimed at disrupting the healthcare space by increasing efficiency, transparency and reducing cost. Investors are backing young people, from outside the industry with new ideas and approaches.
To learn more about our perspective on the changing healthcare ecosystem, download this document by clicking here:
Third Party Administrators, Benefits Consultants and Health Plan Sponsors are All Challenged by the Accelerated Pace of Change
Unprecedented Volume of New Medical Advancements
Payers are struggling with how to handle the torrent of new medical technologies and innovations being put into clinical use.
To manage this, for 40 years, the large payers have relied upon proprietary, well-funded and professionally-staffed committees that few TPAs can afford or cost-justify.
Void of Payor-Oriented Research to Evidence Value
Today, new medical innovations must not only be evaluated and assessed for efficacy but also for value, medical outcomes and patient and provider satisfaction.
Almost no payer-oriented research exists to provide payers with the evidence-based support they need to justify coverage.
New Approach and Tools Needed to Evaluate New Innovations
Measuring value, medical outcomes and patient/provider satisfaction – using real-world-evidence – requires new research approaches and tools, and a degree of scale.
Conducting this type/caliber of research is far beyond the mission, resources and capability of all but a few of the mega-TPAs.
New Medical Technologies, Health Innovations,
Attitudes, Approaches and Ways of Doing Business
Here is a list of just some of the new medical technologies and emerging health innovations that the Research Consortium can research:
- Precision Medicine and Personalized Care
- Genetic/Genomic (DNA) Testing (Diagnostic, Prognostic, Rx)
- Medical Cannabis and Cannabidiol (CBD)
- Medical Wearables / Wireless Monitoring
- Capsule Endoscopes
- Bluetooth-Enabled Smart Inhalers
- Tele-Nutritionist and Tele-Dietitian Consultations
- Remote, High-Risk Patient Monitoring (RPM)
- Nutrigenomics (i.e., which foods to eat and to avoid)
- Non-Invasive Liquid Biopsies
- Companion Diagnostic (CDx) and Biomarkers
- Neuroscience-Based Wearable to Treat Obesity
- 3-Dimensional Printed Body Parts (stents, casts, bones)
- NeuroAD Therapy System (to treat mild Alzheimer's)
- Scalp Cooling (to reduce chemotherapy hair loss)
- Implantable Neuromodulation Device (re Sleep Apnoea)
- Medications to Treat Hearing Loss
- Virtual Primary Care and Robotic Check-Ups
- Regenerative Treatments for Orthopedic Injuries
- Needle-Free Drug Delivery Technologies
- Advanced Implantable and Bioresorbables Devices
- Embracing New Attitudes Toward Health Data Ownership
- Using EMR, Lab and Radiology Data in Plan Management
- Facilitating Patient Contributions to Medical Research
The Research Consortium will also conduct research to reassess commonly employed yet questionable treatments such as dialysis, chemotherapy, spinal injections, hormone replacement, elective C-sections, PSA screening, tests (CT scans, ultrasounds and pre-op tests), etc.
Even Pre-Pandemic, We Needed to Conduct More Medical Research... albeit with a Broken Clinical Research System Needing an Overhaul
We are Living Longer,
but Getting Sicker
While we are certainly living longer, we are also getting sicker. As evidence of this, the number of people age 65 and older having four or more health conditions is expected to double over the next fifteen years.
This tax on our healthcare system will create a need to find new ways to more effectively and efficiently research, understand and manage disease..., ergo, the need and demand for more medical research.
We Urgently Need More
Medical Research
Over the next five years, we in the U.S. will increase our investment in medical research and development to $200+ billion, an increase of 30% in fewer than ten years.
Despite this investment, during the past five years we have seen only half as many new molecular entities (medications) reach the market than in the previous five years. To keep up with the demand for new drugs, these results must be greatly improved.
Failure of the
Clinical Trial Processes
The clinical trial process is broken with low study participation rates, high abandonment and termination rates, enormous time delays and extraordinary financial waste. Indeed, little has been done to change the medical research process in the more than 70 years since it was established.
Our medical research process needs to be reinvented to accommodate the very urgent need for additional medical research.
A Slow Motion Crisis is Brewing in Medical Research and it is Impacting All of Us by Slowing Critical Healthcare Innovation

Half of the New Drugs We Need are Not Being Developed
In the past five years less than half as many new drugs have gotten to market versus the previous five years. Less than 10% of the drugs that start the clinical trials process eventually earn FDA approval.
Many Research Studies Suffer from Low Study Participation
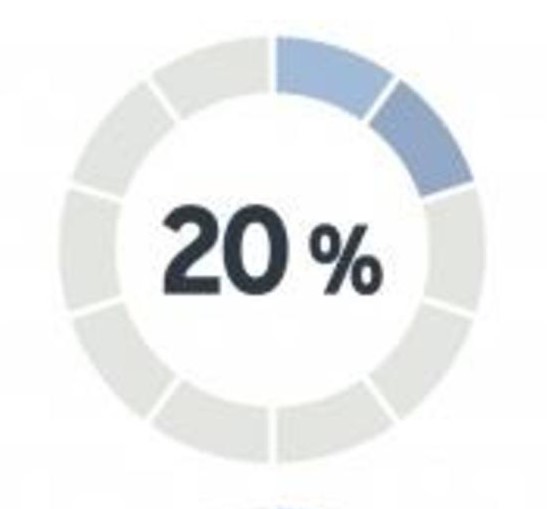
Nearly 20% of all clinical research studies are prematurely terminated due to participation shortfalls. A whopping 80% of all research study sites are unable to recruit sufficient numbers of study participants.
We are Unable to Recruit
Research Study Participants
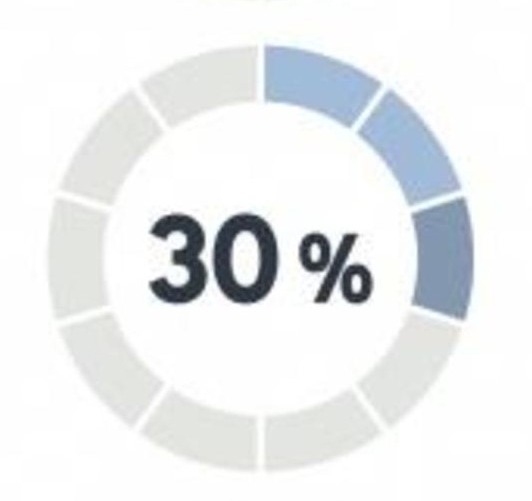
We need 50+ million research participants to complete the 125,000 studies already underway in the U.S. alone…and have less than 30% of the research participants we need to populate these studies.
Although the Demand for Participants is Great, and Patients are Abundant, the Cost of Clinical Research Recruiting is Exorbitant
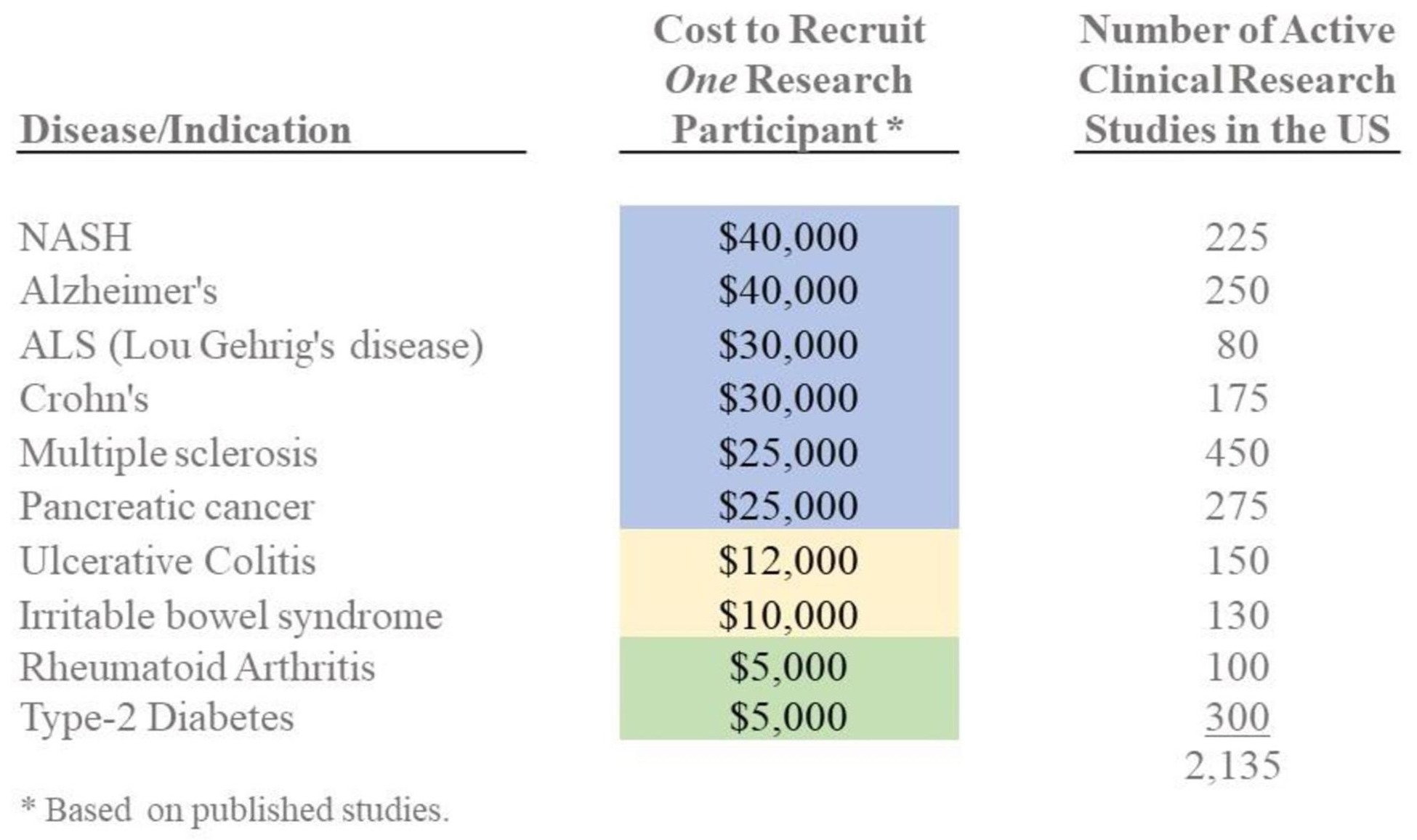
On average, it is estimated that it costs more than $15,000 to recruit one patient for one phase of a typical clinical trial. In large part, this expense is a result of our inefficiency at educating physicians and patients about research opportunities; identifying suitable study participants; communicating effectively about research participation opportunities; and operational problems that make physician participation a burden rather than a worthwhile endeavor. All of these very correctable problems greatly impact the amount of research conducted and the development cost of bringing new medical technologies and innovations to market.
The Good News: Patients are Interested in Learning More About and Participating in Clinical-Research-as-a-Care-Option Programs...
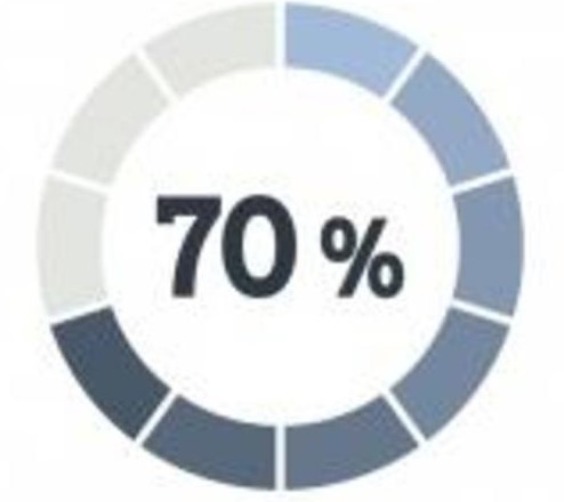
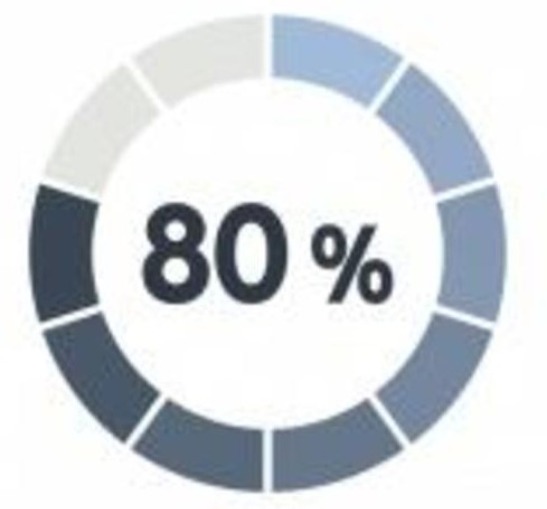
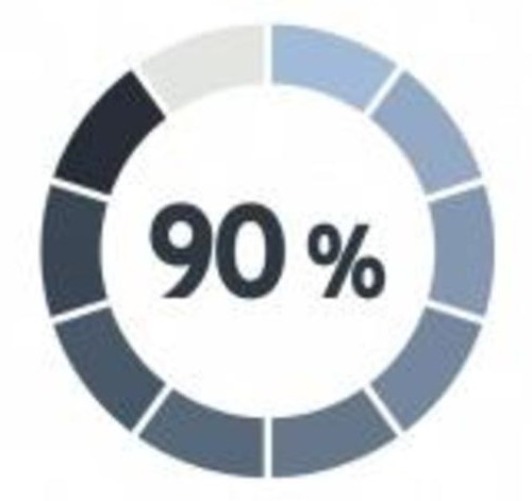
Patients are Willing to Participate in Research
Recent surveys indicate that more than 70% of respondents would be either "interested" or "very interested" in participating in a medical research study if recommended to do so by their doctor; unfortunately most doctors and patients are not so informed.
Patients Want to Know About Research Opportunities
National surveys show that ~ 80% of respondents believe it’s important to be made aware of the chance to participate in medical research and more than 85% believe that study participation opportunities should be a routine part of standard care.
Patients are Very Satisfied with Participating in Research
Multiple research study participant surveys evidence a 90% patient satisfaction level, improved medical knowledge; significantly better or improved quality of care; reduced overall health care costs; and decreased complications and hospital admissions.
...and Patients are Very Willing to Carefully Share Their Healthcare and Medical Data with Medical Researchers and Their Health Plan

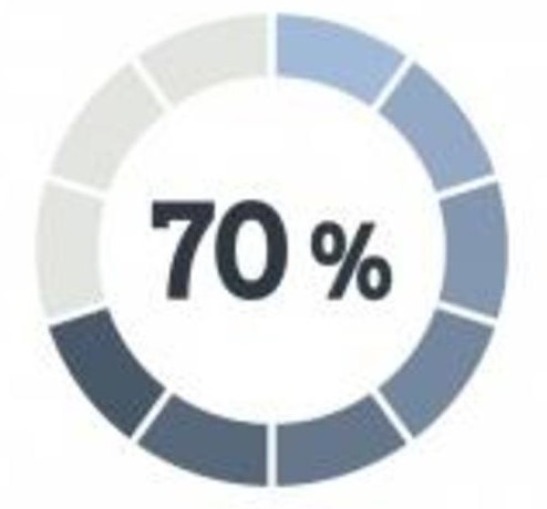
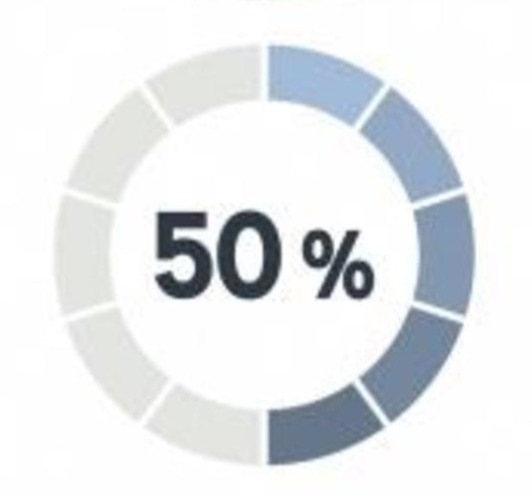
Patients Willingness to Share Medical Data with Researchers
More than 70% of survey respondents said they were willing to selectively share their medical data with certain research groups.
Patient Interest in Obtaining Actionable Information
Nearly 70% of survey respondents were most interested in receiving feedback about potential risk factors gleaned from their data.
Patient Willingness to Share Medical Data with Health Plan
More than 50% of the patients willing to share their medical data with researchers for insight would share it with their plan.
The Research Consortium performs research of value to Payers and Plan Sponsors; helps Plan Participants access leading-edge medical care; and provides Medical Researchers with valuable data and access to clinical research study participants. Our mission is to harness and direct the vast resources of the self-funded and commercial payer communities in ways that help to accelerate the adoption of emerging medical technologies and reduce the time it takes to bring new health innovations from the work bench into widespread clinical use...something that now take more than 15 years on average.
The Research Consortium focuses on issues that are of
interest and value to everyone in the healthcare ecosystem.
Ready to Learn More About the Research Consortium ?
.
